Overcoming Chemistry Hurdles: Insights from a Top Chemistry Tutor in Brisbane

Understanding Basic Chemistry Concepts
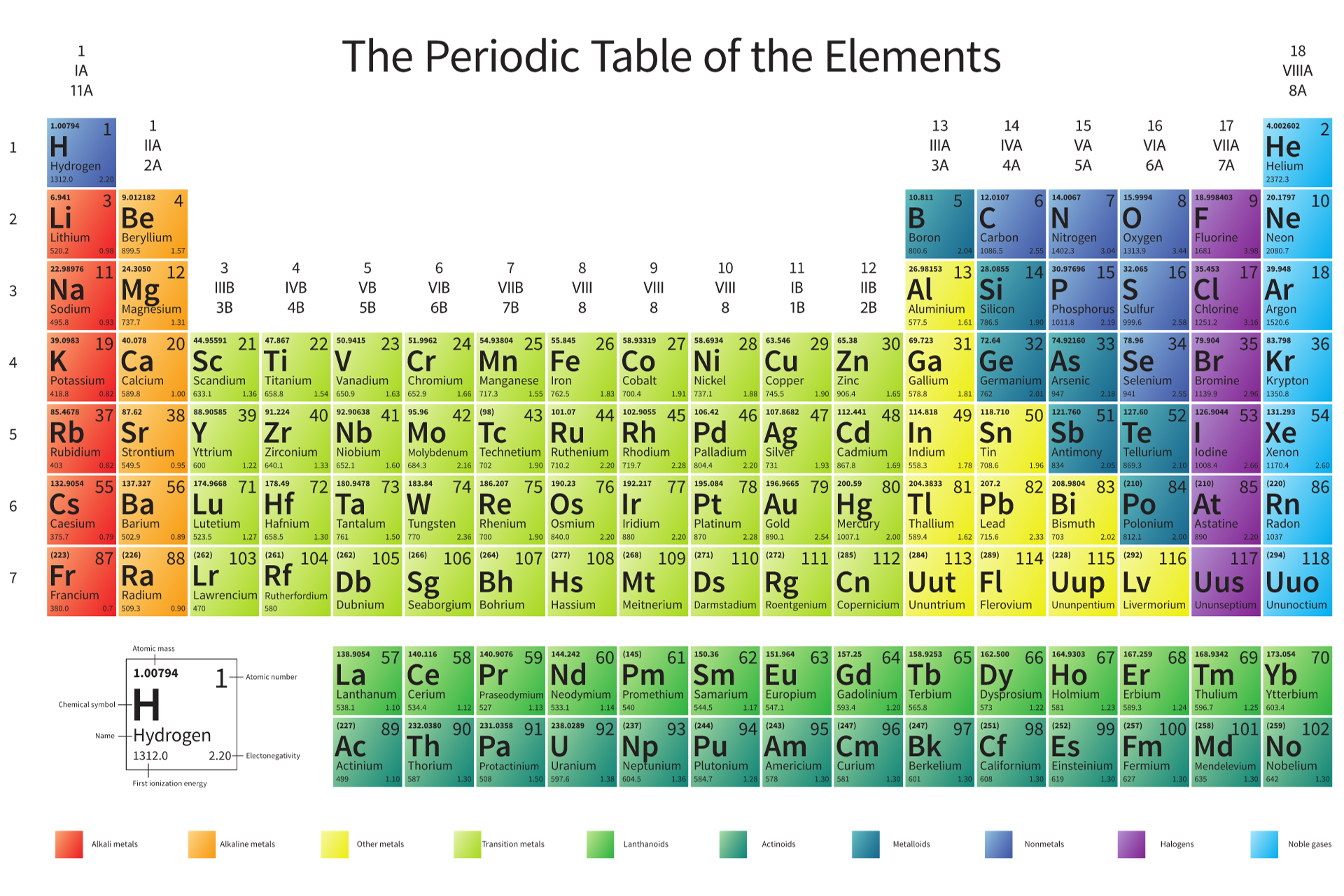
Atomic Structure and the Periodic Table
The foundation of chemistry starts with understanding atomic structure and the periodic table. Atoms are like tiny building blocks that make up everything around us. Each atom has a nucleus (the centre) with protons and neutrons, and electrons that orbit around this nucleus. Sounds simple, right? But how these atoms interact with each other is what chemistry is all about.
The periodic table is your roadmap in chemistry. It’s not just a bunch of boxes with letters; it’s a well-organised system that tells you loads about the elements and how they behave.
Here’s a tip: start by getting familiar with the first 20 elements. Know their names, symbols, and a few properties. Fun activities like flashcards or mnemonic devices can make this learning a breeze.
Chemical Bonding Basics
Now, let’s talk about how atoms stick together – that’s chemical bonding for you. There are mainly two types: ionic and covalent. Ionic bonding happens when atoms transfer electrons, usually between a metal and a non-metal. Picture it like a give-and-take relationship. Covalent bonding, on the other hand, is more about sharing electrons, and this usually happens between non-metals. Think of it as a team effort where both atoms share to achieve stability.
Understanding these bonds is crucial because they determine the properties of a substance. A good way to grasp this is through simple experiments or models.
For example, using balls and sticks to model different types of bonds can be both fun and educational. Remember, the more you play around with these concepts, the more you’ll understand them.
Mastering Chemical Reactions and Equations
Balancing Chemical Equations
Balancing chemical equations might seem a bit like a puzzle at first, but it’s actually a fundamental skill in chemistry. Every chemical equation needs to be balanced to show that mass is conserved during a reaction. That means the number of atoms for each element should be the same on both sides of the equation.
Here’s a simple step-by-step guide to get you started:
- Write the Unbalanced Equation: Start with the correct formulae for the reactants and products.
- Count the Atoms: Look at how many atoms of each element are on each side of the equation.
- Balance One Element at a Time: Use coefficients (big numbers in front of the formulae) to balance the atoms. Start with the element that appears in only one reactant and one product.
- Repeat and Check: Keep adjusting the coefficients until all elements are balanced. Then, double-check your work.
A common pitfall is forgetting to balance polyatomic ions (like NO3 or SO4) as a whole.
Also, remember that coefficients change the amount, but subscripts (the little numbers in the formulae) are part of the formula and can’t be changed.
Types of Chemical Reactions
Understanding different types of chemical reactions helps you predict what happens in a reaction. Here are a few basic types with real-world examples:
- Synthesis Reactions: Two or more substances combine to form a new compound. For instance, when iron and sulfur are heated together, they form iron sulfide, a new compound.
- Decomposition Reactions: A compound breaks down into simpler substances. Think of how electrolysis breaks down water into hydrogen and oxygen gas.
- Single Replacement Reactions: One element replaces another in a compound. An everyday example is when zinc reacts with hydrochloric acid, zinc replaces hydrogen, producing zinc chloride and hydrogen gas.
- Double Replacement Reactions: The ions in two compounds exchange places to form two new compounds. An example is when you mix silver nitrate with sodium chloride, producing silver chloride and sodium nitrate.
Each reaction type has its own set of rules and patterns. By understanding these, you can start to predict the products of reactions and make sense of why certain reactions occur. Real-world examples like these make the concepts stick better and show how chemistry is part of our everyday life.
Having struggles with home schooling? Get the help you need!
Demystifying Stoichiometry
Introduction to Stoichiometry
Stoichiometry might sound like a complex word, but it’s actually a really handy part of chemistry. It’s all about the quantities in chemical reactions. In simpler terms, stoichiometry tells you how much of each substance is needed or produced in a reaction. It’s like a recipe in cooking: how much of each ingredient do you need to make the perfect dish?
Understanding stoichiometry starts with a balanced chemical equation. This equation not only tells you which substances react and what products are formed but also gives you the ratios in which they react and are produced. For basic stoichiometry calculations, remember these tips:
- Start with a Balanced Equation: You need to know the correct ratios of reactants and products.
- Convert to Moles: Stoichiometry deals with moles (a chemistry unit that measures the amount of a substance).
- Use the Mole Ratio: Use the coefficients from the balanced equation to set up ratios for the reactants and products.
- Solve for the Unknown: Use the mole ratios to find out how much of one substance is needed or produced if you know the amount of another.
Accuracy is key in stoichiometry, so double-check your calculations and make sure you understand each step.
Real-Life Applications of Stoichiometry
Stoichiometry isn’t just for the lab – it has loads of practical uses in everyday life. For example, in cooking, stoichiometry helps in tweaking recipes. If you know the basic chemistry of baking, you can adjust ingredients to get the perfect cake or bread.
In the environment, stoichiometry plays a role in understanding pollution. Scientists use stoichiometry to figure out how pollutants react in the atmosphere or water, helping us tackle environmental challenges.
To really get a grip on stoichiometry, practice is key. Let’s look at a simple practice problem:
Problem: If 2 moles of hydrogen react with oxygen to produce water, how many moles of water are produced?
Solution: The balanced equation for the reaction is 2H2 + O2 → 2H2O. This tells us that for every 2 moles of hydrogen, 2 moles of water are produced. So, if we start with 2 moles of hydrogen, we’ll produce 2 moles of water.
Tackling Organic Chemistry
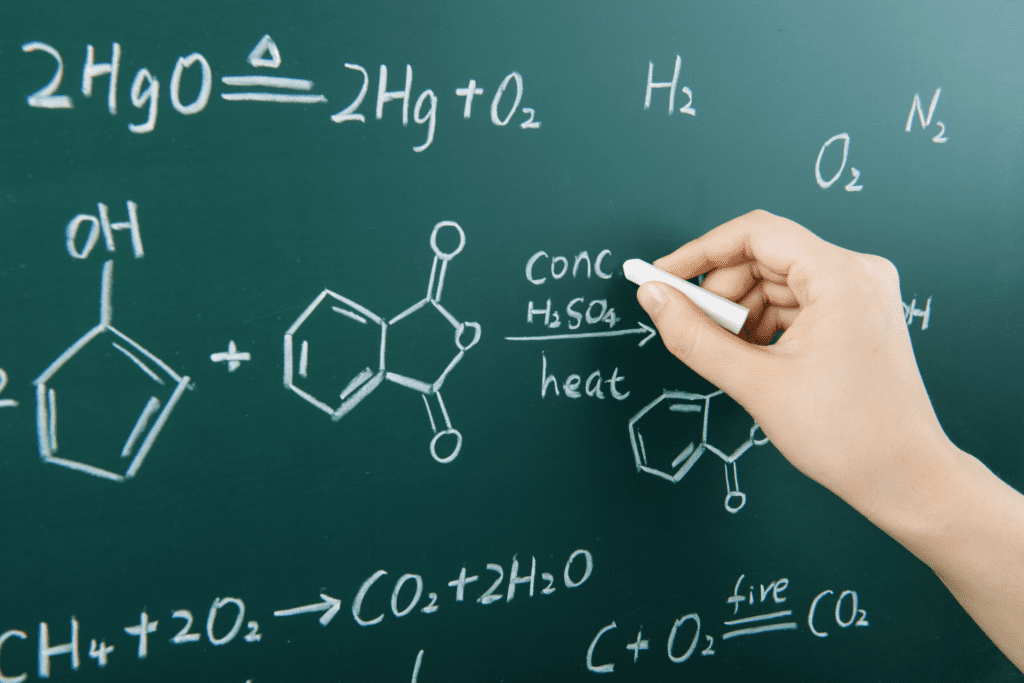
Understanding Organic Compounds
Organic chemistry, the study of carbon-containing compounds, is a vast and fascinating field. The key to starting off right in organic chemistry is to understand the naming of organic compounds and the role of functional groups.
Simplifying Organic Nomenclature:
- Organic compounds are named based on the carbon chain length, the type of bond (single, double, or triple), and the functional groups they contain.
- The length of the carbon chain forms the base of the name (like methane, ethane, propane), and the type of bond adds a prefix or suffix (like -ene for double bonds, -yne for triple bonds).
- Learning the basic rules of nomenclature, such as numbering the carbon chain to give the lowest numbers to the functional groups, can simplify the process significantly.
Functional Groups and Their Properties:
- Functional groups are specific groupings of atoms within molecules that have their own characteristic properties.
- For instance, alcohols (with an -OH group) are known for their solubility in water, while carboxylic acids (with a -COOH group) are known for being acidic.
- Understanding these properties helps predict how different organic compounds will behave and react.
Basic Reaction Mechanisms
Decoding Organic Reactions:
- Organic reactions often seem overwhelming, but understanding the basics of reaction mechanisms can demystify them.
- Each reaction mechanism involves the breaking and forming of bonds in a step-by-step sequence.
- Visualizing these steps, either through drawing mechanisms or using molecular models, can be incredibly helpful.
Tips for Mastering Reaction Pathways:
- Focus on Electron Movement: Track how electrons move during reactions, as this often dictates the course of the reaction.
- Pattern Recognition: Many organic reactions follow similar patterns or sequences. Recognizing these can help you anticipate the products of new reactions.
- Practice with Examples: Apply what you learn to real-world examples. Try to predict the products of a reaction before looking them up.
- Mnemonics and Analogies: Use memory aids or everyday analogies to remember complex reactions. For example, think of a substitution reaction as swapping seats in a game of musical chairs.
Organic chemistry can initially seem like a maze of complex reactions and names, but with these strategies, it becomes more like a puzzle to be solved. By breaking down concepts, using visual aids, and practicing regularly, you’ll find that organic chemistry is not only manageable but also quite fascinating.
Acids, Bases, and pH
Exploring Acidity and Basicity
Acids and bases are everywhere – in the food we eat, the cleaning products we use, and even inside our bodies. Understanding them starts with the pH scale, which measures how acidic or basic a substance is. The pH scale ranges from 0 to 14, with lower numbers being more acidic, higher numbers more basic, and 7 being neutral (like pure water).
Understanding the pH Scale:
- The pH scale is logarithmic, which means each number on the scale represents a tenfold change in acidity or basicity. For example, a pH of 4 is ten times more acidic than a pH of 5.
- The pH of a solution is determined by the concentration of hydrogen ions (H+) it contains. More H+ ions mean a lower pH (more acidic), and fewer H+ ions mean a higher pH (more basic).

Tips for Calculating pH:
- To calculate pH, you can use the formula pH = -log[H+], where [H+] is the concentration of hydrogen ions in moles per liter.
- Understanding the relationship between acidic or basic solutions and their pH is crucial. For example, if you know a solution is acidic, you can predict its pH will be less than 7.
Practical Applications
Everyday Examples:
- Acids and bases are part of daily life. Lemon juice and vinegar are common acids, while baking soda and soap are basic.
- These substances can interact in useful ways. For example, baking soda (a base) can neutralize vinegar (an acid), which is why they’re often used together in cleaning.
Exploring Acidity and Basicity Through Experiments:
- Simple experiments can help you understand acids and bases. For instance, you can use red cabbage juice as a pH indicator. This natural indicator changes color depending on the pH of a substance.
- Another fun activity is to experiment with neutralization reactions. Mix a small amount of baking soda with vinegar and watch the reaction. The fizzing is a result of the acid (vinegar) and base (baking soda) reacting to form carbon dioxide gas.
Understanding acids, bases, and the pH scale not only makes chemistry more interesting but also helps you make sense of the world around you. Whether it’s figuring out why certain foods taste sour or how cleaning agents work, a good grasp of these concepts can be incredibly useful.
Chemistry Success: Putting It All Together
As we come to the end of our chemistry exploration, it’s clear that with the right approach and understanding, chemistry can be less daunting and more fascinating. We’ve covered a lot, from the basics of atomic structure and the periodic table to the complexities of organic reactions and the intriguing world of acids, bases, and pH. Now, let’s briefly recap what we’ve learned and think about how you can use these tips in your own studies.
- Get to Grips with the Basics: Start with a solid understanding of atomic structure, chemical bonding, and the periodic table.
- Balance Those Equations: Remember the importance of balancing chemical equations and understanding different types of reactions.
- Stoichiometry is Your Friend: Embrace stoichiometry for its practical applications and problem-solving prowess.
- Organic Chemistry Unraveled: Tackle organic chemistry by breaking down nomenclature, functional groups, and reaction mechanisms.
- Acids and Bases Demystified: Explore the pH scale and everyday examples of acidity and basicity.
Chemistry is everywhere in our lives, and understanding it can be incredibly rewarding. We encourage you to apply these tips and insights in your studies. Remember, practice and persistence are key. The more you engage with these concepts, the more they’ll make sense.
Need a Helping Hand? Tutor Wombat is Here!
Sometimes, a bit of extra help can make all the difference. At Tutor Wombat, we understand the challenges and joys of learning chemistry. Our experienced chemistry tutors in Brisbane, and all over Australia, are here to provide personalized support. Whether you’re grappling with the basics or diving into more advanced topics. We’re committed to helping you achieve chemistry success, tailored to your unique learning style and pace.
Chemistry doesn’t have to be a mystery. With Tutor Wombat’s personalized tutoring, you can master the subject and even enjoy the journey. Ready to take your chemistry understanding to the next level? Contact Tutor Wombat to find out more about our tutoring services and how we can support your chemistry adventure.
So go ahead, dive into the fascinating world of chemistry, and see how these tips and strategies transform your approach and understanding. With Tutor Wombat by your side, you’re well on your way to becoming a chemistry whiz!



